[Global Research Trends] 8. Leading Companies and Countries with Top Publications on Drug Approval Post-Covid (2021-2024)
![[Global Research Trends] 8. Leading Companies and Countries with Top Publications on Drug Approval Post-Covid (2021-2024)](/content/images/size/w1200/2024/10/hand-medical-glove-holding-vaccine-vial-1.jpg)
/ Scinapse Trends Series #8
From this week, ‘Scinapse Trends’ will be releasing bi-weekly on Mondays instead of Fridays. Begin your week with cutting-edge research trends and insights from industry experts at Scinapse ! 😊
Understanding trends in drug approval research has become increasingly important as the field undergoes rapid change, especially since the COVID-19 pandemic. The pandemic significantly reshaped the drug development and approval process, impacting not only the speed at which new treatments reach the market but also how we evaluate them.
Why Drug Approval Research Is Important? - Before and After COVID-19
- Before COVID-19, healthcare systems worldwide were already facing high levels of pressure due to numerous chronic diseases and increasingly complex therapeutic needs.
- New types of treatments, from traditional small molecules to biologics and mRNA vaccines, required more specialized and advanced approval processes tailored to each unique treatment.
In recent decades, drug approval research has evolved considerably. There has been a growing emphasis on advanced clinical trial design and real-world evidence, moving beyond a pure safety focus. Regulatory agencies now consider broader benefit-risk assessments that include patient-reported outcomes and adaptive trial designs. The rise of precision medicine has also paved the way for more personalized therapies requiring tailored approval paths.
Before 2020, drug development often took over a decade due to the step-by-step nature of traditional processes. However, the pandemic forced regulatory bodies to adapt quickly. Instead of waiting for complete data from each phase, COVID-19 encouraged parallel processes and quicker data assessments to maintain research continuity and improve efficiency. The success of rapid vaccine development during the pandemic showed that with urgency and resources, development timelines can be shortened without sacrificing safety.
Many anticipated a return to “business as usual” post-pandemic, but instead, these changes have remained. Regulatory agencies, such as the FDA and EMA, have incorporated tools developed during the pandemic, like remote clinical trials and real-time data assessment, into regular drug applications. This adaptation wasn’t solely about speed; it was a response to urgent public health needs.
The rapid development and approval of COVID-19 vaccines and treatments set a new standard, not just for future health crises but for routine drug development as well.
In this Scinapse Trends series, we will explore the publication trend during post-pandemic, from 2021 to 2024 shortly, to see which countries and especially each company have conducted the most research in this field.

Publication Trends on Drug Approval and Top Publication Countries & Commercial Companies (2021-2024)
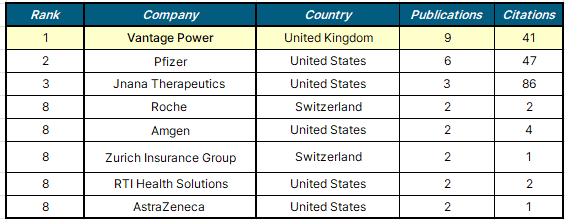
Among top affiliations, excluding universities, hospitals, and research institutions, the UK-based commercial company Vantage Power ranked first with 9 publications. Although Vantage Power primarily focuses on designing and manufacturing innovative battery packs, its life sciences subsidiary, Evaluate Vantage, frequently publishes research reports and analyses on pharmaceutical industry trends and drug approvals. These reports also include data on regulatory shifts post-pandemic and evaluate various therapies, such as small molecules, biologics, and cell therapies. Through comprehensive analysis, they help stakeholders make strategic resource allocations and prioritize decisions based on market trends, emerging therapeutic areas, and projected sales potential.
Following Vantage Power, U.S. companies like Pfizer and Jnana Therapeutics occupied the second and third positions. Companies such as Amgen, RTI Health Solutions, and AstraZeneca shared ninth place, including well-known names like Pfizer and AstraZeneca, which significantly contributed to post-COVID drug approval trends by adopting new methodologies and regulatory flexibility introduced during the pandemic.
Additionally, the Swiss global healthcare brand Roche and Zurich Insurance Group also held joint 9th place with 2 publications each. Notably, the 5th-ranking Jnana Therapeutics achieved high-quality research outcomes with 86 citations, reflecting strong research impact.
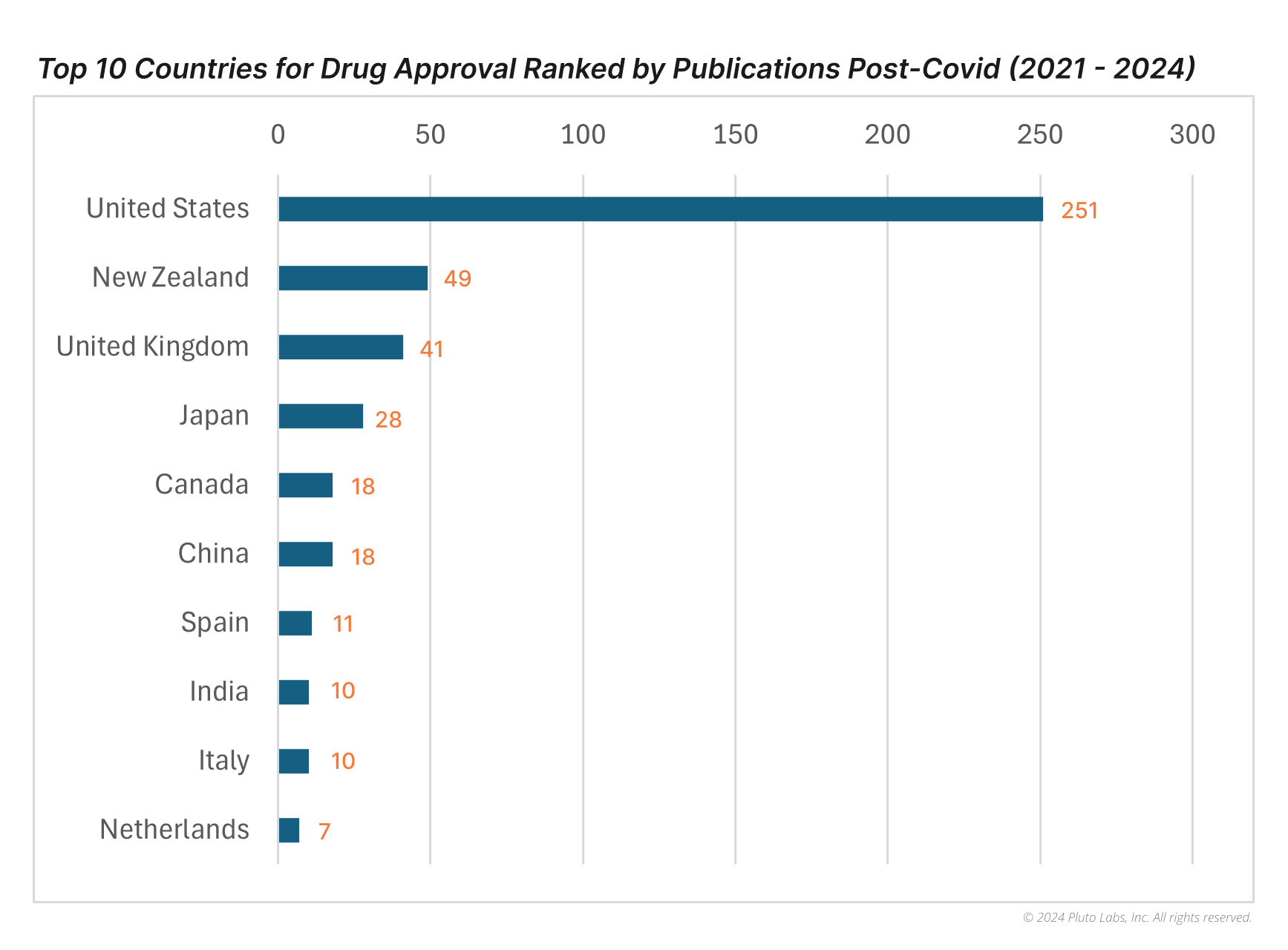
Based on the graphs and data above, the United States ranks first with an overwhelming 258 publications in 3 years, far outpacing other countries—nearly 6 times more than New Zealand's 49 publications in 2nd place and the United Kingdom's 41 in 3rd. Japan follows in 4th with 28 publications, while Canada and China tie for 6th with 18 publications each.
In terms of qualitative research performance with research impact, New Zealand leads with an average of 5.96 first-year citations, significantly higher than the United States (2.24), the United Kingdom (2.44), and Spain (4.18). This is largely due to Lesley J. Scott’s publication, Lumasiran: First Approval, affiliated with Springer Nature, which has an impressive 133 citations.
Note: 2024 data is only partially reflected, and "Top Affiliation" is based on the lead author's last (the most recent) affiliation.
How Has Drug Approval Evolved Over the Past Years?
Originally, Scinapse's Advanced Research Intelligence Filter provided research summaries for the past 3, 5, and 7 years. However, since the period covered this time is focused on the last 3 years, it offers concise research insights focusing on this shorter timeframe.
Over the past 3 years, the research has focused on analyzing FDA drug approvals, including novel drugs, accelerated approval programs, and COVID-19 vaccines, as well as comparing regulatory pathways between the EU and the US.
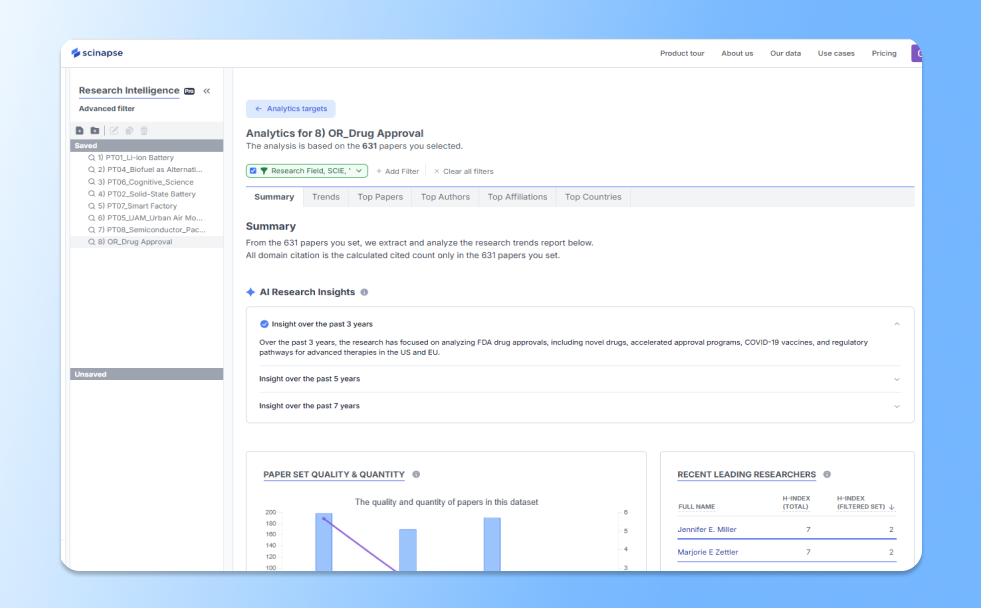
The “AI Research Insights✨” shows how research has been conducted and progressed within your search criteria over the last 3, 5, or 7 years.
How Can I Extract Research Insights From Scinapse?
Scinapse's Research Intelligence Advanced Filter allows users to add and edit various filters according to their research objectives. This enables searching for the right paper and gaining insights into advanced research trends.
You can view the most concentrated results by starting with the Summary tab, where a summary of the desired results is displayed. In the Top Countries tab, you can see publications and average first-year citations by country, and in Top Affiliations, you can also view the h-index of each affiliation.
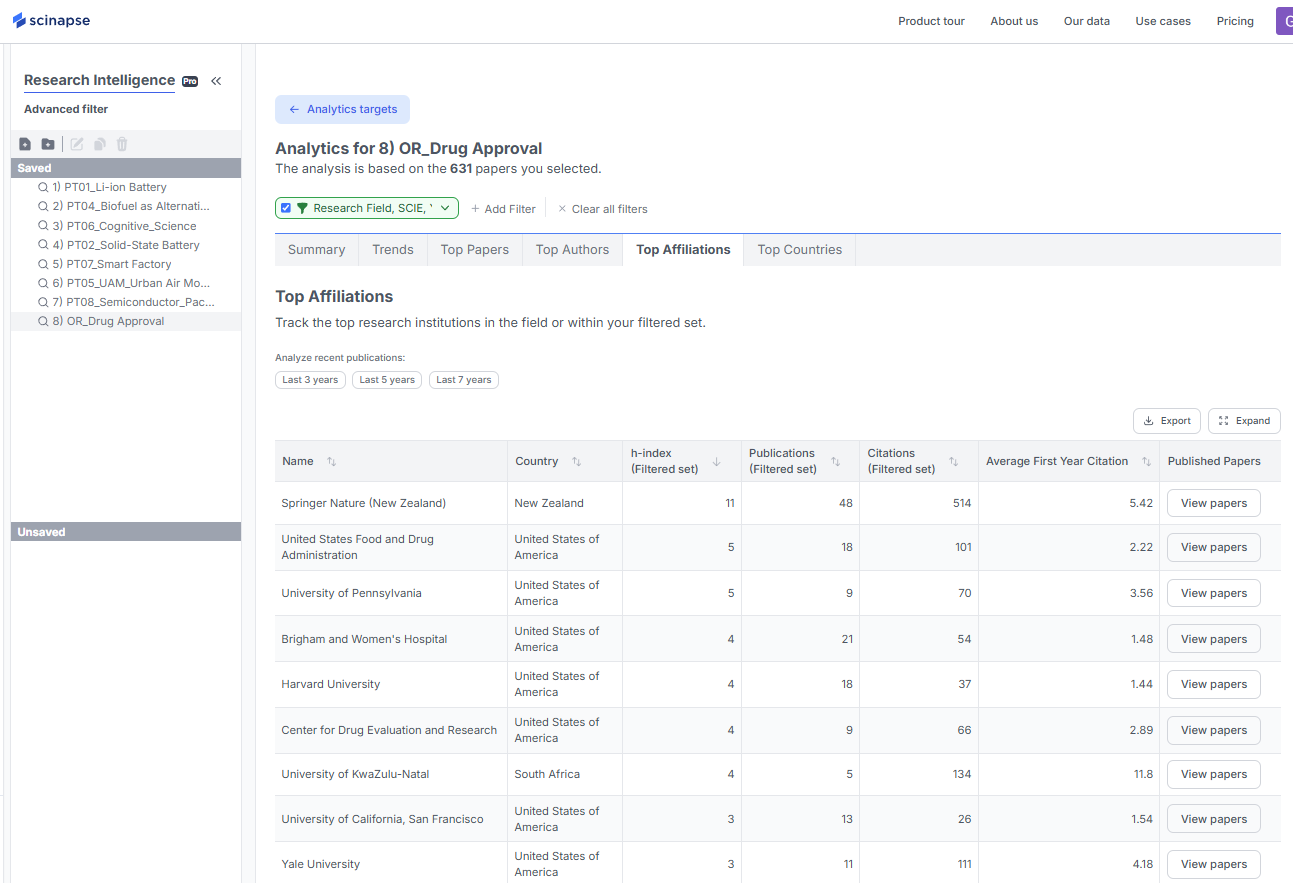
(However, Scinapse does not offer a feature to differentiate between the nature of institutions.)
👉🏻If you need more information from Research Intelligence, Try it Now!
👇🏻Need more insights? Book a demo today!
/ Data source - Scinapse
/ Written by Geehee Nahm
- Content Strategist specializing in AI and SaaS industries
For Previous Scinapse Trends 🔽


![[Global Research Trends] 10. Top 10 Countries on Microneedle Research Publications (2014-2024)](/content/images/size/w720/2024/11/Scinapse-Trends_10.png)
![[Global Research Trends] 9. Top 10 Countries on FOWT Research Publications and Authors' Normalized h-Index (2014-2024)](/content/images/size/w720/2024/11/Scinapse-Trends_9--1-.png)
Comments ()